2.2 The total energy balance: exercise 1 (example)
Course subject(s)
2. Energy balances
Exercise 1
MAKING TEA
Imagine that you want to make some tea. To do this, you heat up some water in a kettle on your gas stove in the kitchen. In this exercise, you will calculate how much energy is required when heating up a kettle of water and how much energy a combustion reaction can deliver to a gas stove.

You will need a pen and paper for this exercise. A calculator would help as well. Here you can find the formula sheet.
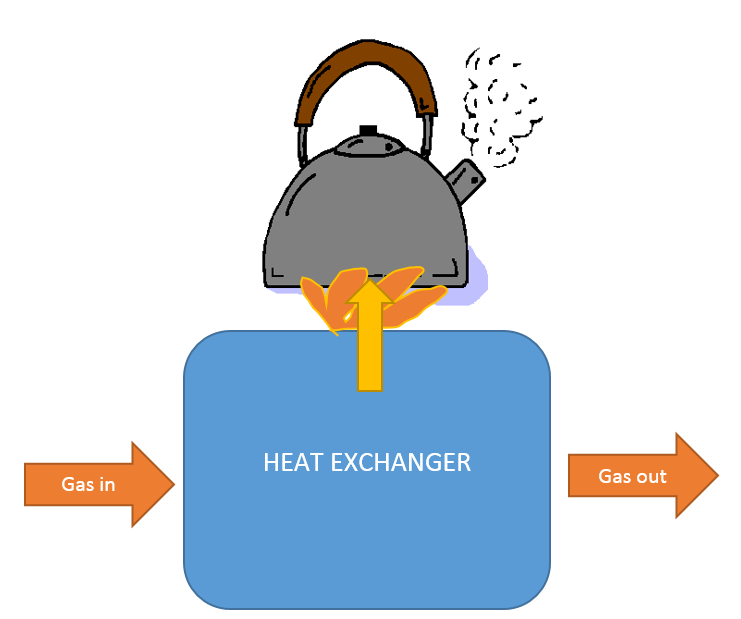
The kettle filled with water is put on the gas stove and you have to wait until the water boils and evaporates. In this situation, a stream of heat is supplied to the kettle and a jet of hot steam leaves it.
In this example, the fuel gases of the gas stove are methane (CH4) and air (20% oxygen and 80% nitrogen) at 20 °C. You can see this in Figure 1. The place where the methane is burned is called a heat exchanger. The released heat will be extracted from the gasses. The measured temperature of the outflow, which is a combustion product (all gases), is 120 °C. The pressure is constant in the whole system. You can take a molar heat (cp) of 40 J/(mol∙K) for all gases.
The reaction of burning methane is as follows: CH4+2O2 -> CO2 + 2H2O.

The Basics of transport phenomena by TU Delft OpenCourseWare is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Based on a work at https://ocw.tudelft.nl/courses/basics-transport-phenomena/.



