2.2 The total energy balance: examples
Course subject(s)
2. Energy balances
Examples
EXAMPLE 2.2A. HEATING UP WATER – PART I (ELEMENTARY)
You would like to have a cup of tea, so you need to heat up water. In this exercise, you do that by putting water (0.5 liter) in a cooker that is electrically heating up its content.
The water out of the tap is 20oC. The question here is: how long does the cooker need to be switched in order for the water to reach a temperature of 80oC?
Negelect the heat capacity of the cooker. Furthermore, ignore heat losses to the surroundings
The specific heat of water is 4200 J/kgK. The electric power of the cooker is 1kW.
- Do a simple calculation without using a balance.
- Redo the calculation. But now set up a heat balance over a proberly chosen control volume. Solve the balance and find the required time.
Sorry but there don't seem to be any downloads..
Subtitles (captions) in other languages than provided can be viewed at YouTube. Select your language in the CC-button of YouTube.
EXAMPLE 2.2B. HEATING UP WATER – PART II (MEDIUM)
In the previous problem, we analyzed how long it takes to warm up water from 20oC to 80oC, ignoring heat losses to the environment. That is, obviously, a symplification of reality. In this exercise, we revisit the same problem, but now consider heat losses as well.
The question is: how long does it take to heat up 0.5 liters of water that is initially at 20oC? The water is inside a cooker that has a power of 1kW.
The heat flow from the water to the surroundings is modelled as ϕq=β(T−T∞), with β=5.3W/K and T∞=20∘C the temperature of the surroundings.
- Set up a thermal energy balance for the water, taking into account the heat loss.
- Solve the balance and find the required time. It should be longer than the time in EQ 2.1A.
Sorry but there don't seem to be any downloads..
Subtitles (captions) in other languages than provided can be viewed at YouTube. Select your language in the CC-button of YouTube.
EXAMPLE 2.2C: HEATING UP WATER – PART III (ADVANCED)
In the previous two exercises, we lookead at the heating up of water in a cooker. First, we did a very rough estimate, ignoring any forms of heat losses. In the second question, we took heat loss into account via a simple relation to the water temperature.
In this exercise, we will consider what happens when the water is actually boiling.
The question is: how long does it take for 0.5 liters of water that is boiling at 100oC to be completely evaporated? The water is inside a cooker that has a power of 1kW.
- Make a ‘quick & dirty’ approach without using balances.
- Make an analysis using the balance concepts:
- Make a sketch with the relevant transport phenomena
- Set up a mass balance for the liquid water
- Set up an energy balance for the liquid water. Actually, an enthalpy balance is most suited. If you are unfamiliar with this, don’t worry. Just try and check the solution and see if you can understand what is done.
- Solve these balances and compute the required time
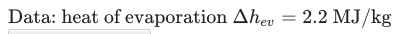
Sorry but there don't seem to be any downloads..
Subtitles (captions) in other languages than provided can be viewed at YouTube. Select your language in the CC-button of YouTube.

The Basics of transport phenomena by TU Delft OpenCourseWare is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Based on a work at https://ocw.tudelft.nl/courses/basics-transport-phenomena/.



