4.1.6 Example A: Partition coefficient
Course subject(s)
4. Mass transfer
We have a column filled with air, that is contaminated with a hydrocarbon component. How can we remove this hydrocarbon component? We take solvent droplets with a diameter of ![]() that are falling down in the air. So, the hydrocarbon component will go to the interface of the solvent and will dissolve into the droplet. To describe this, we have to know how this hydrocarbon is distributed between the air and solvent phase. If we look to the interface the concentration in the air phase is 4 times more than in the solvent phase, so
that are falling down in the air. So, the hydrocarbon component will go to the interface of the solvent and will dissolve into the droplet. To describe this, we have to know how this hydrocarbon is distributed between the air and solvent phase. If we look to the interface the concentration in the air phase is 4 times more than in the solvent phase, so 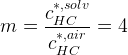 .
.
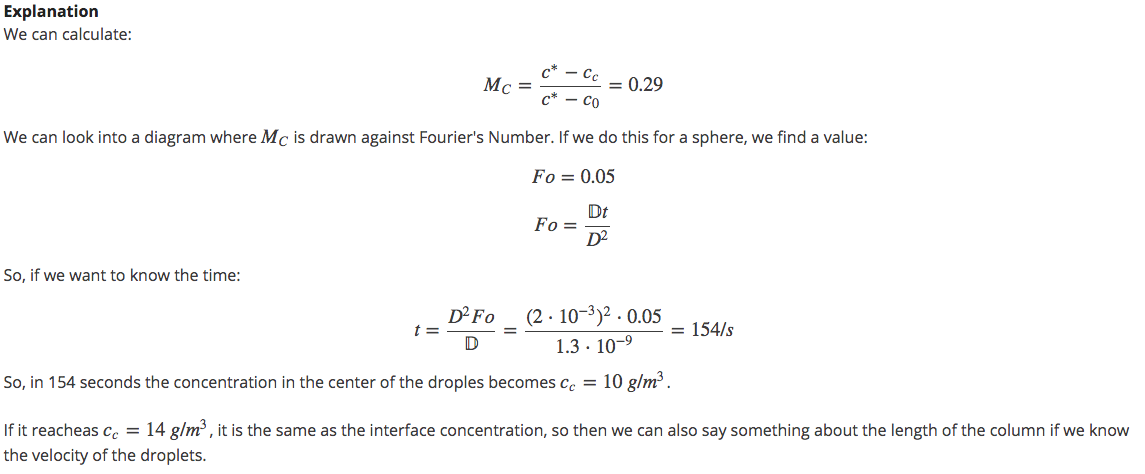
We also take a look at the concentration distribution in the solvent droplet. How long does it take before the concentration in the center of the droples reaches ![]() ? In the beginning
? In the beginning ![]() and
and ![]() . The resistance for mass transfer is inside the droplet and the diffusion coefficient is
. The resistance for mass transfer is inside the droplet and the diffusion coefficient is ![]() .
.
Sorry but there don't seem to be any downloads..
Subtitles (captions) in other languages than provided can be viewed at YouTube. Select your language in the CC-button of YouTube.

Advanced Transport Phenomena by TU Delft OpenCourseWare is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Based on a work at https://online-learning.tudelft.nl/courses/advanced-transport-phenomena/.



