5. Elaboration Question 8
Course subject(s)
5. Nitrogen and phosphorus removal
Question 8: Chemical phosphorus removal with lime
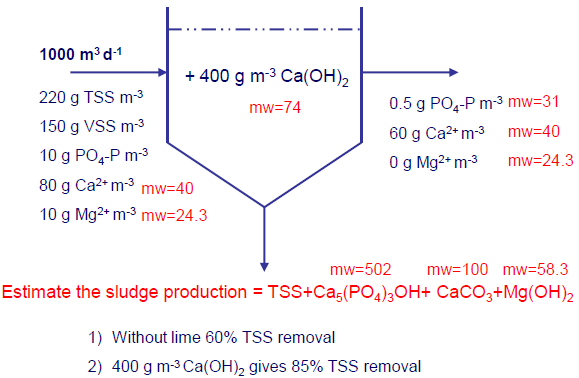
Estimate the mass and volume of sludge produced in a primary sedimentation tank from the precipitation of phosphorus with lime. Assume that 60% of the TSS is removed without the addition of lime, and that the addition of 400 mg/l of Ca(OH)2 results in an increased removal of TSS to 85%. Assume the following data apply:
- Wastewater flowrate = 1000 m3/d
- Wastewater Total Suspended Solids (TSS) = 220 mg/l
- Wasteater Volatile Suspended Solids (VSS) = 150 mg/l
- Wastewater PO43- as P, mg/l = 10 mg/l
- Wastewater total hardness as CaCO3 = 241.3 mg/l
- Wastewater Ca2+ = 80 mg/l
- Wastewater Mg2+ = 10 mg/l
- Effluent PO43- as P, mg/l = 0.5 mg/l
- Effluent Ca2+ = 60 mg/l
- Effluent Mg 2+ = 0 mg/l
- Chemical sludge properties
- Specific gravity = 1.07
- Moisture content= 92.5%

Urban Sewage Treatment by TU Delft OpenCourseWare is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Based on a work at https://ocw.tudelft.nl/courses/urban-sewage-treatment/.



